My 2.5-year journey with translocation renal cell carcinoma
April 30, 2024
This is a guest post by Dawn Dyson, 37, who was diagnosed with translocation renal cell carcinoma in 2022. Dawn…
Read More
Below are some highlights from research presented at the 2023 meeting of the American Society of Clinical Oncology, which took place in Chicago, Illinois from June 2-6.
Cabozantinib plus nivolumab is a promising treatment for advanced papillary kidney cancer
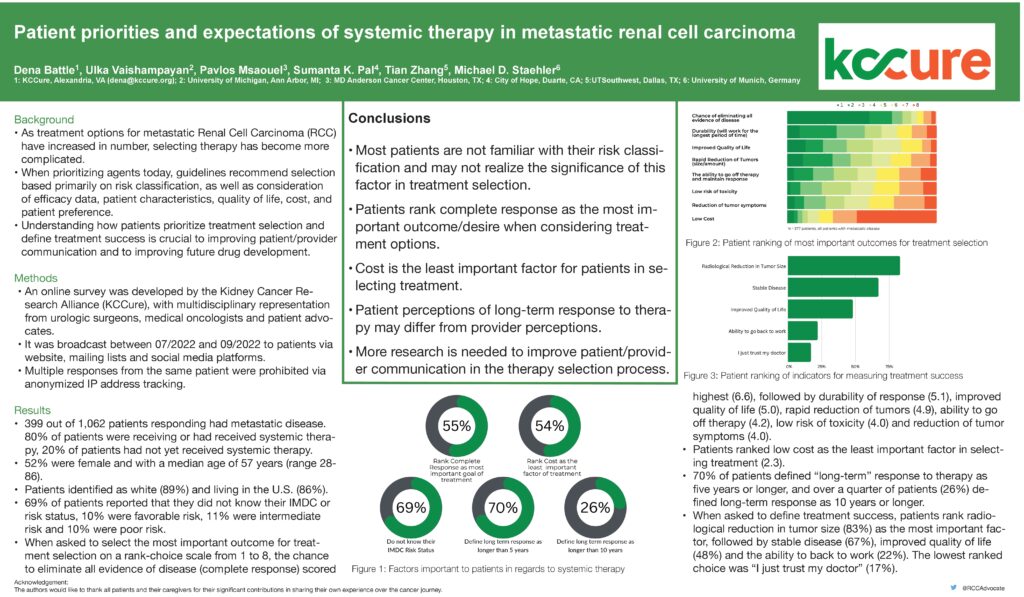
Dr. Chung-Han Lee, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York and member of the KCA’s Medical Steering Committee, presented two phase two studies on promising treatments for advanced non-clear cell kidney cancers. One built upon the phase 2 CaboNivo study, published in 2022, assessing the efficacy and safety of cabozantinib plus nivolumab in patients with non-clear cell renal cell carcinoma (RCC). The combination was most effective in cancers with papillary features while chromophobe RCC showed limited effects.
The updated results focus on a cohort of 40 patients, most of whom had metastatic papillary kidney cancer. Nearly two thirds of them were previously untreated while a third had received targeted therapy.
Nearly half of the patients responded to treatment with an average of 13 months till treatment stopped working. Half of the patients showed no progression through 1 year; a quarter showed no progression through 2 years. 70% of patients survived for 18 months and 44% for 3 years. There was no difference in survival for previously treated and untreated patients.
Most patients (88%) reported a side effect, with just over half reporting serious or life-threatening side effects, such as liver damage, high blood pressure and pain. Treatment was stopped in 28% of patients because of side effects. The combination treatment also did not work for a cohort of chromophobe RCC patients and was closed early. However the results highlight the efficacy of cabozantinib plus nivolumab in patients with metastatic non-clear cell kidney cancer, especially metastatic papillary kidney cancer. [ASCO 2023, Abstract 4537]
First-line lenvatinib plus pembrolizumab controlled disease in advanced non-clear cell kidney cancer
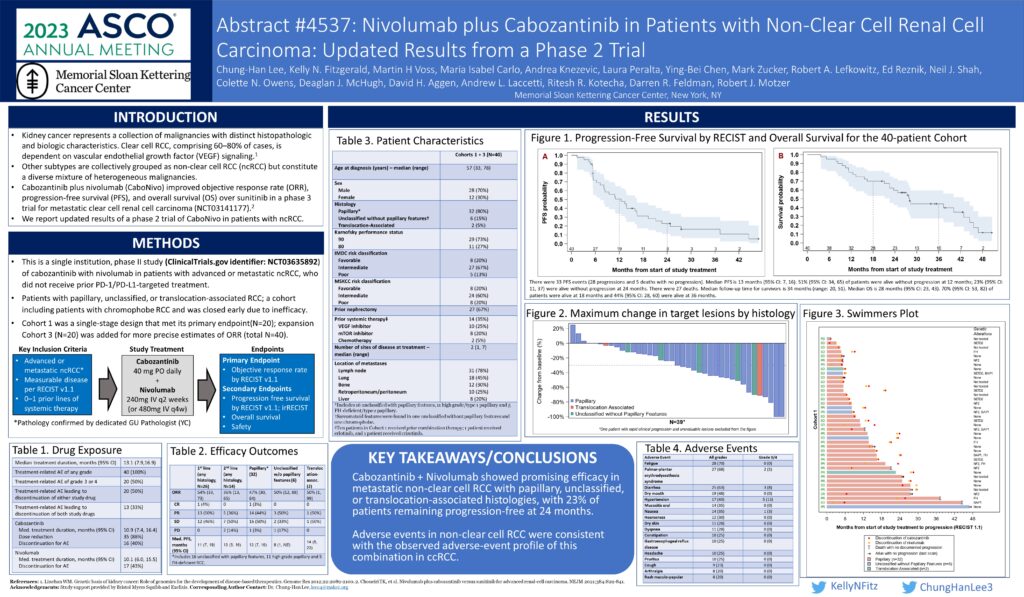
The second study led by Dr. Lee was a follow up to the phase 2 KEYNOTE-B61 trial in which the combination of lenvatinib plus pembrolizumab proved effective as a first-line treatment for advanced clear cell kidney cancer and early results showed that the combination is also effective as a first-line treatment for advanced non-clear cell kidney cancer.
There were 158 patients in the study. Most patients had papillary kidney cancer (59%). A fifth of patients had chromophobe kidney cancer, and a few had translocation kidney cancer (4%) or another subtype (6%). The subtype of kidney cancer was not known for the remaining patients (13%). Patients were followed for nearly 15 months.
Half of the patients responded to treatment and the cancer was controlled in 82% of patients. Three quarters of patients had a response that lasted for at least one year. The response rate was similar for patients with different cancer risks (favorable, intermediate, and poor risk). The average time to when the treatment stopped working and the cancer started growing again was 18 months for all patients. Responses were seen in all subtypes of kidney cancer included in the study.
The side effects were like those previously reported for the combination of lenvatinib plus pembrolizumab. Side effects were reported by 94% of patients. The most common side effects were high blood pressure, diarrhea, and hypothyroidism. About half of the patients reported serious or life-threatening side effects. Only 3% stopped treatment with the combination because of side effects.
This study shows that the combination of lenvatinib plus pembrolizumab is an effective first-line treatment for patients with different subtypes of non-clear cell kidney cancer. [ASCO 2023, Abstract 4518]
Quality of life questionnaires for kidney cancer patients need an overhaul.
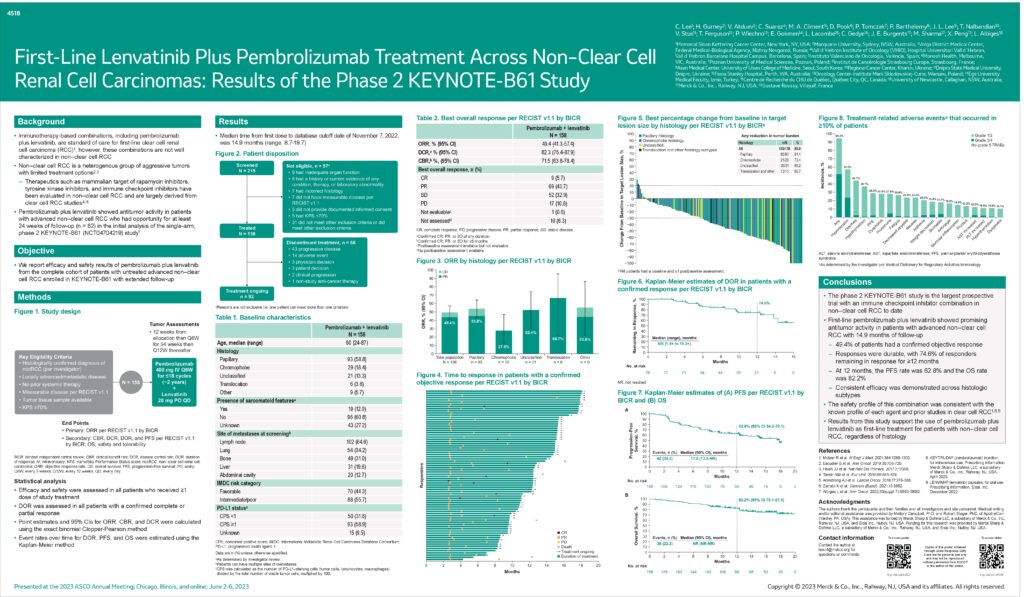
Balancing survival with quality of life is important for people with advanced kidney cancer. However, a new research survey of kidney cancer patients presented at ASCO showed that most of them did not find existing quality-of-life questionnaires used for cancer patients (the FKSI-19, EORTC QLQ-C30, and EQ-5D) to have relevant questions. The survey, conducted by Dr. Cristiane Bergerot of the Oncoclinicas in Brazil, included 116 patients from Brazil and the US. Most (69%) were male, and the average age was 64. 83% were diagnosed with advanced kidney cancer, and three quarters had received immunotherapy and/or targeted therapy as their first anti-cancer treatment. Few of the questions in all the questionnaires were considered meaningful to the patients. Those considered meaningful were questions about lack of energy, fatigue, appetite, sleep, worry, ability to work, enjoyment and quality of life, and overall health. Patients suggested including questions about treatment side effects, emotional symptoms, physical function, social/family support, and financial distress. 58% of patients were open to using wearable devices to assess health-related quality of life.
Dr. Bergerot previously received a 2021 KCA Psychosocial Focus Award for research that contributed to this study, now in its second phase, which confirms that health-related quality of life questionnaires for kidney cancer patients need major changes. The findings from this study will be used to help develop a new kidney cancer health-related quality of life questionnaire in collaboration with patients. [ASCO 2023, Abstract 4540]
What patients know about kidney cancer treatment
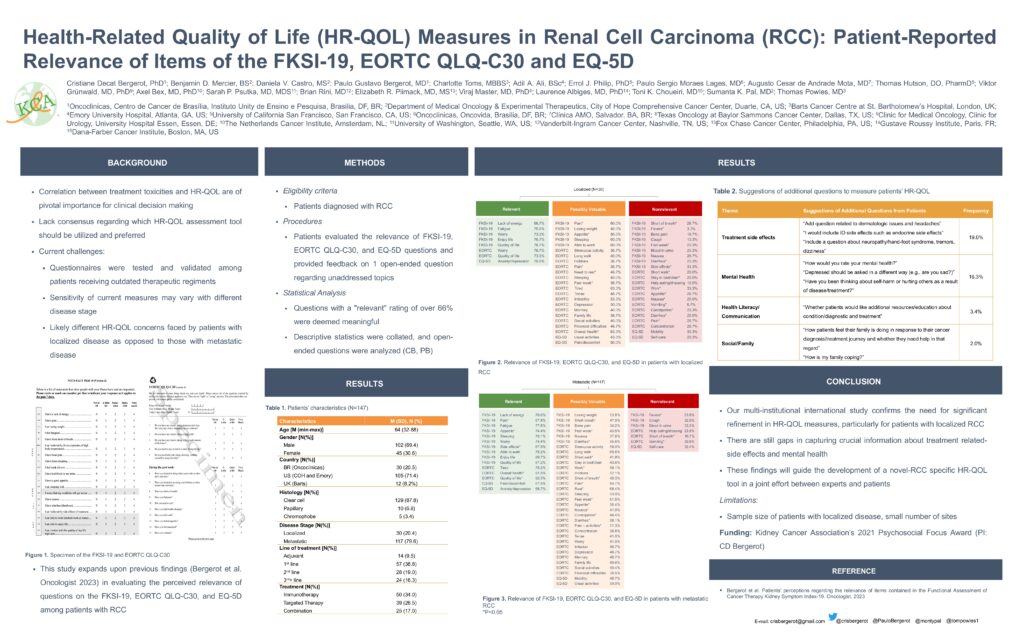
A survey of over 1000 kidney cancer patients found that most patients do not know the risk of their disease and may not realize how their risk impacts their treatment choices. Respondents reported that complete response is the most important aspect of treatment while cost is the least important. However, patients’ interpretation of “long-term response” differs from that of their doctors’.
Patients were asked to rank the most important outcome for the selection of treatment on a scale from 1 through 8. The chance to eliminate all evidence of disease (a complete response to treatment) scored highest (6.6), followed by the length of the response (5.1), improved quality of life (5.0), rapid reduction of tumours (4.9), ability to come off therapy (4.2), low risk of side effects (4.0) and reduction of tumour symptoms (4.0). Patients ranked low cost as the least important factor in selecting treatment (2.3).
A total of 70% of patients defined long-term response to treatment as five years or longer, and over a quarter of patients (26%) defined long-term response as 10 years or longer. When asked what a successful treatment is, most patients (83%) said a reduction in the size of the tumour on a scan is most important, followed by stable disease (67%), improved quality of life (48%) and the ability to return to work (22%).
Around 40% of patients in the survey had kidney cancer that has spread. 80% of these patients had already been treated with an anti-cancer medication. More than two thirds of patients (69%) did not know their risk for kidney cancer. The remaining third were split equally between favorable, intermediate, and poor risk.
Treatment options for kidney cancer that has spread (metastatic kidney cancer) have increased over the past 10 years. Selecting treatment has become more complicated. Treatment guidelines recommend selecting treatment based mostly on kidney cancer risk, as well as the effectiveness of the treatment, patient characteristics, quality of life on treatment, cost, and patient preference. More research is needed to improve communication between the patient and their doctor when deciding on treatment options. [ASCO 2023, Abstract 4560]