
Heather Fought Through a Rare Diagnosis for Motherhood
May 12, 2024
This is a guest post by Heather Williams, 25, an ER nurse who was diagnosed with renal medullary carcinoma. She…
Read More
By D’Ann George, PhD, Medical Writer
This year’s American Society of Clinical Oncology meeting in June featured the first-ever symposium devoted to microbiome research in cancer. The “Role of the Microbiome in Immune CheckPoint Inhibitor Response and Resistance” session featured two speakers of particular interest to people with metastatic renal cell carcinoma (mRCC).

Mixed Phase 2 Results in mRCC Microbiome Trial
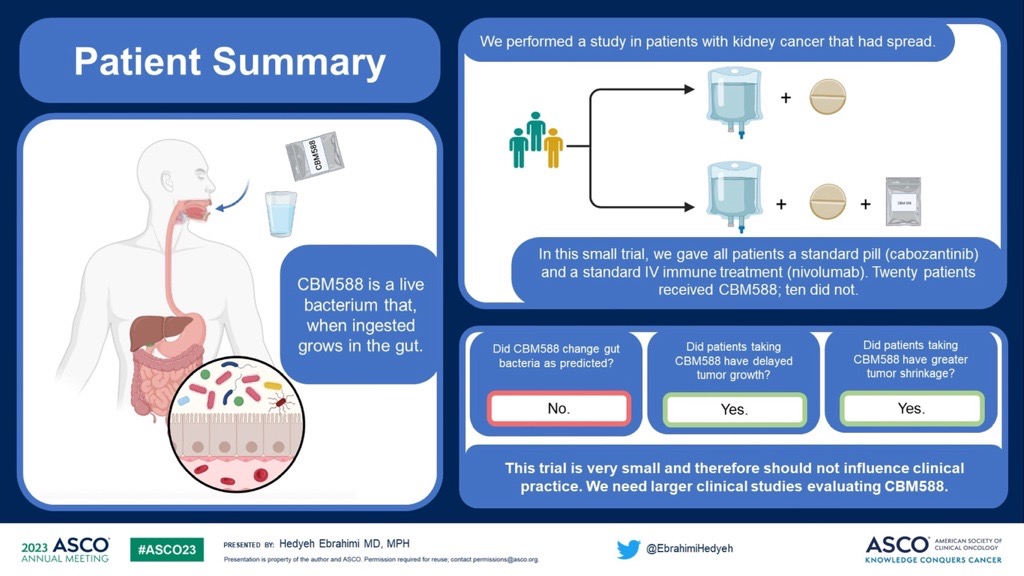
Dr. Hedyeh Ebrahimi, a kidney cancer researcher at City of Hope Comprehensive Cancer Center, reported results of a small phase 2 clinical trial at her institution that tested the relative effectiveness of a triplet therapy combining a live bacterial product (CBM588) with a standard regimen for mRCC (nivolumab plus cabozantinib) versus the standard regimen alone. [ASCO 2023, Abstract LBA 104]
Participants in the experimental group received the triplet therapy while the control group received the standard nivolumab plus cabozantinib regimen.
The results of the trial were mixed. The CBM588 study failed to meet either of its primary objectives. Twelve weeks into the study, people assigned to the experimental group did not show increased gut bacterial diversity, as hypothesized, nor did they show an increase in the bifidobacterium species, another prediction.
Increasing bifidobacterium was a goal for the study because past research demonstrated that mice supplemented with this microbe improved their response to anti-PD1 immune checkpoint inhibitor therapies like pembrolizumab and nivolumab, said Dr. Shahla Bari, a medical oncologist and expert on microbiome research in RCC at Duke Cancer Institute, who was not involved in the study.
However, trial participants in the experimental arm did meet the secondary objective of improved progression-free survival (PFS) compared to the control group. Specifically, the group of people taking the triplet had yet to reach a median PFS, meaning that the majority of people in this group are still alive and have not progressed.
In contrast, the median or 50th percentile PFS for the people in the control group was just 5.8 months.

According to Bari, survival results from the control group were worse than expected based on the CHECKMATE 9ER study of the standard therapy.
“I’m cautious when interpreting these results. The CBM588 study was conducted in a small group of patients, and that limitation should be taken into account,” she said.
Ebrahimi emphasized the limitations of her study results, too, citing the small sample size (30 participants) and that the experimental group had a slightly higher proportion of people with favorable-risk factors.
Dr. Thomas Gajewski, an oncologic pathologist at the University of Chicago Medicine who was not involved in the study, led the formal discussion and response to the CBM588 trial data.
While he called the relatively higher PFS of the experimental arm encouraging, he also pointed out the lack of a clear mechanism of action that would cause people ingesting CBM588 to have a more favorable response.
“I didn’t see data yet on what changes in the immune microenvironment were happening in the tumor post-treatment,” he said.
Ebrahimi said that a cooperative group is considering a phase 3 trial of CBM588, but she doesn’t know how soon a trial might begin or who would be eligible to participate.
Microbiome as a Potential Biomarker for RCC
At the same session, Dr. Lisa Derosa, a medical oncologist at the Institut de Cancerologie Gustave Roussy in Paris, France discussed a complex new biomarker pioneered by her research team in a French/Canadian prospective study. The trial evaluated whether the gut microbiota composition of people undergoing immunotherapy treatment for cancer correlates with their prognosis, overall survival, and PFS. [ASCO 2023, Abstract 103]
She said the novel biomarker, a calculated ratio of gut microbiota called TOPOSCORE, predicted overall survival in metastatic RCC participants more accurately than did traditional International mRCC Database Consortium (IMDC) prognosis risk scores. Clinical trials in RCC use the IMDC score to sort participants into experimental and control arms.
Derosa’s research team, who developed the biomarker, tested TOPOSCORE first in people with melanoma, then later validated their results by testing the marker in people with three other types of solid tumors: RCC, urothelial, and non-small cell lung carcinoma.
In less than 48 hours from the time microbial samples are taken, TOPOSCORE can predict the effectiveness of a person’s response to an immune checkpoint inhibitor. TOPOSCORE works by detecting the relative abundance of bacteria that aid the immune system in fighting off cancerous cells to those that destroy the immune system’s ability to stop attacks on itself.
The French group identified 34 different types of gut bacteria that predicted an overall survival of greater than one year and 40 different species that predicted an overall survival less than one year.
In addition to predicting who will respond well to immunotherapies, TOPOSCORE could select donors and recipients of fecal microbial transplantations, Derosa said.
Session discussant Dr. Jennifer McQuade, a medical oncologist at MD Anderson Cancer Center in Houston, Texas, who was not involved in either of the presented trials, reflected on the significance of Derosa’s presentation by providing a brief history of microbiome research in cancer.
A trio of publications in the journal Science in 2018 pointed to an association between the gut microbiome and response to immunotherapy. The exciting discovery led to many more studies across the United States and Europe looking for gut microbes that could predict response to immunotherapy, but most of them revealed the challenges of trying to use any single microbe as a biomarker.
“Something that works very well in the UK does not work well in the Netherlands, even though . . . this was the same exact sequencing and analyses. And when they apply this same learning as well to other cohorts around the world, again, actually poorly predictive,” said McQuade.
“Maybe the issue is we shouldn’t just be looking at single bugs. We need to look at ecosystems. The microbiome exists as an ecosystem. There are interdependencies . . . There are functional redundancies. There are significant interactions with the host, and the environment, and specifically, with diet dependencies that might vary country to country . . . it may actually be easier to define a bad microbiome rather than a good one, which is what Dr. Derosa’s test would do.”
McQuade does not believe that TOPOSCORE is ready for clinical application.
“I would suggest that, for now, we’re still learning about this score and working on further validation,” she said.
McQuade also does not believe that TOPOSCORE–or any microbiome biomarker–will ever be used as a stand-alone predictor for immunotherapy response. Instead, she said the field is headed toward predictive algorithms that have a combination of clinical, molecular, and microbiome features.
For now, she said a tool like TOPOSCORE could be used as a stratification factor in microbiome-directed trials, ensuring that each cohort in a clinical trial has equal numbers of people with favorable and unfavorable gut bacteria.
Future Directions for Microbiome Research in Kidney Cancer
A general lack of consensus among researchers about which gut microbes are associated with immune checkpoint inhibitor blockage (ICB) complicates the way forward for researchers. Bari said part of the confusion lies in the apparent redundancy of the microbiome.
“Different microbial species can serve the same function among individuals,” she said.
“One solution in the future may be for research to focus on the functions of the microbiota, rather than just the microbiota itself. The microbial metabolome in addition to the microbiome could be developed as a stronger predictor of response to immunotherapy.”
Bari remains enthusiastic about the field of microbiome and microbial metabolome research in kidney cancer.
“It may be possible one day to modulate microbial metabolic pathways and improve immunotherapy response.”